Alright, let's dive into the cosmic kitchen where stars are the chefs and elements are the dishes. Stellar nucleosynthesis is like a grand cosmic cooking show, with stars whipping up batches of elements in their fiery cores. It's the process that cooks up all the elements in the universe, from the hydrogen in your water bottle to the gold in your jewelry.
Imagine the Big Bang as the grand opening of the universe's kitchen. It left us with a pretty simple menu: mostly hydrogen, a dash of helium, and a sprinkle of lithium. But as any chef will tell you, simplicity is just the starting point for something amazing. Stars took these simple ingredients and started cooking up new elements in their core.
Now, how do they do it? Well, stars are hot. I mean, really hot. The core of a star is like the ultimate pressure cooker, with temperatures and pressures so extreme that atoms get all cozy and fuse together. When they do, they form heavier elements in a process called nuclear fusion. This is the heart of stellar nucleosynthesis.
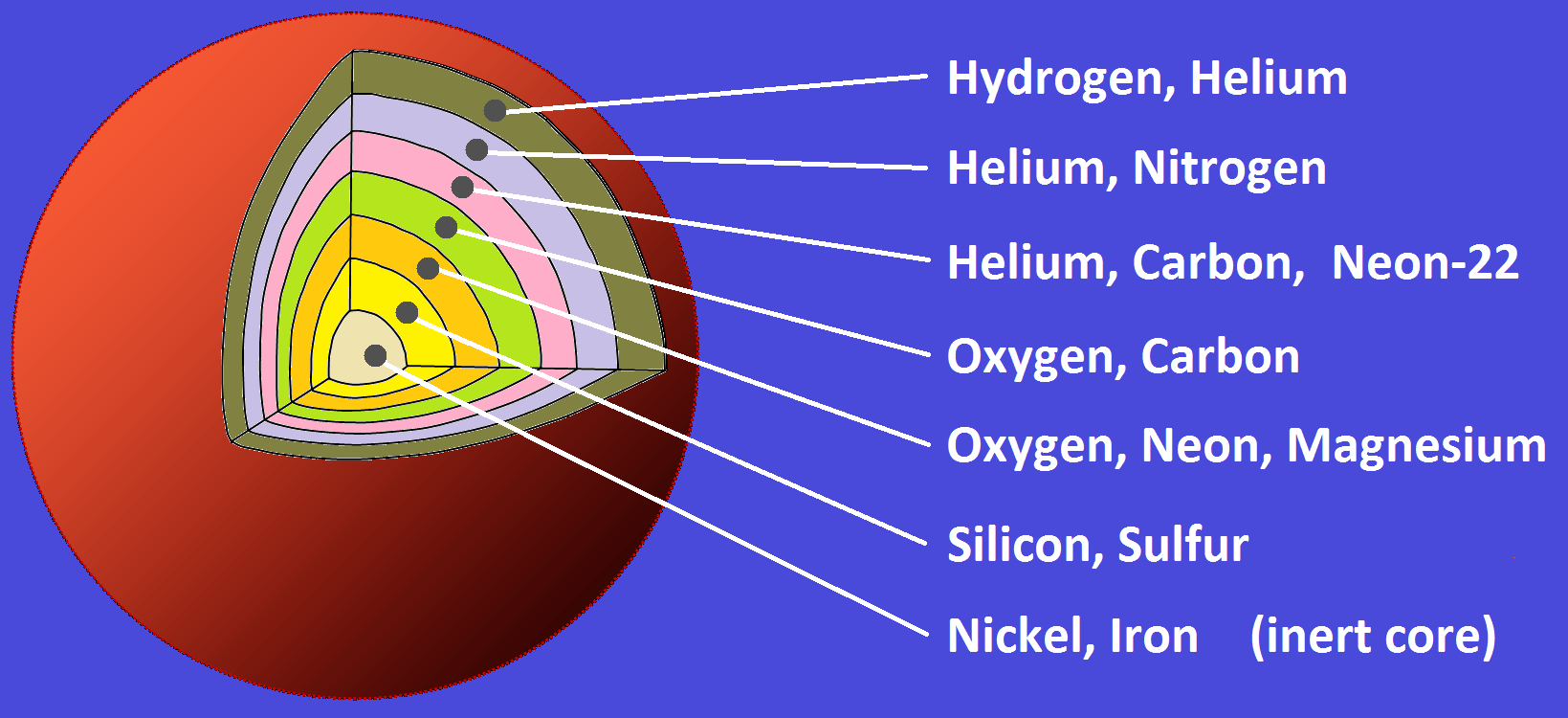
Let's break it down. You start with hydrogen, the simplest element. In the core of a star, hydrogen atoms fuse to form helium. This process releases a ton of energy, which is why stars shine. But stars aren't one-trick ponies. As they age, they start fusing helium into even heavier elements like carbon and oxygen. This is like moving on from making a simple salad to a more complex dish.
The process doesn't stop there. In the most massive stars, the fusion menu becomes even more exotic. They can cook up elements all the way to iron, which is like the culinary limit for fusion. Why? Because fusing iron doesn't release energy—it actually requires energy. And in the world of stars, that's a big no-no. It's like a recipe that costs more to make than it sells for.
But the universe has a trick up its sleeve for creating elements heavier than iron, and it involves some stellar fireworks. When massive stars run out of fuel, they don't just fade away—they go out with a bang in a supernova explosion. This cataclysmic event is so powerful that it creates a pressure and temperature surge, allowing elements to capture neutrons and form all the rest of the elements on the periodic table. It's like a master chef throwing all the spices into the pot at once and creating something entirely new and amazing.
The remnants of these explosions, including all these newly minted elements, get scattered across the cosmos. They become part of nebulae—stellar nurseries where new stars are born. And the cycle begins anew, with the next generation of stars incorporating these elements and cooking up their own.
So, the next time you look at a periodic table, remember that it's more than just a chart—it's a menu of cosmic proportions, with each element telling the story of a star's life. And just like a family recipe passed down through generations, the elements we have today are the legacy of countless stars that have lived and died in the vast expanse of the universe.
Isn't it wild to think that the atoms that make up everything around us, from the air we breathe to the cells in our bodies, were all forged in the heart of stars? We're literally made of star stuff. So, in a way, we're all walking, talking stellar souvenirs.
And there you have it, the story of stellar nucleosynthesis, the cosmic process that's been cooking up elements for billions of years, serving up a universe rich with the ingredients for planets, life, and everything we know.


Share:
The Role of AMOC and Consequences of its Collapse
Plate Boundaries vs. Faults: Understanding Earth's Tectonic Forces